What is FDA Covered Product Authorization (CPA)?
FDA Covered Product Authorization refers to the official approval given by the U.S. Food and Drug Administration (FDA) for certain medical products to be marketed in the United States.
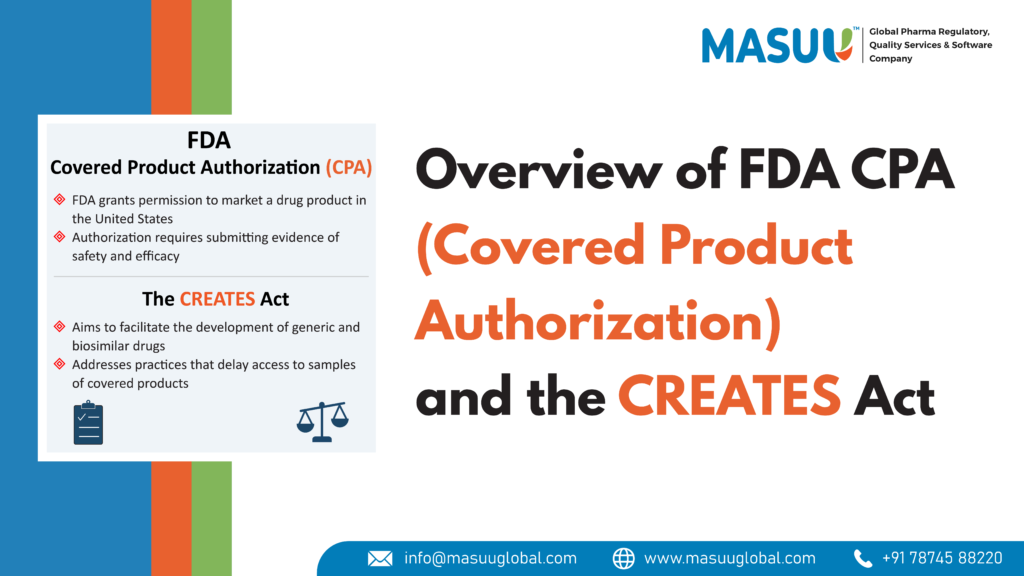
- Prescription drugs
- Biologic products (e.g., vaccines, gene therapy, insulin)
- Medical devices
- Safe for use
- Effective for its intended purpose
- Manufactured according to strict quality standards
These products are protected under FDA regulations and U.S. law, often involving patents and exclusive marketing rights. This ensures that the innovator has a period of commercial benefit before generics or biosimilars can enter the market.
What is the CREATES Act?Creating and Restoring Equal Access to Equivalent Samples Act
It is a U.S. federal law passed in 2019 to promote fair access and competition in the pharmaceutical market.
Purpose of the CREATES Act:To prevent brand-name drug companies from using unfair tactics to block generic and biosimilar drug manufacturers from entering the market.
Generic and biosimilar manufacturers need samples of brand-name (covered) products to conduct testing and FDA-required studies. Some brand-name companies refused to provide samples or used safety regulations (like Risk Evaluation and Mitigation Strategies – REMS) as excuses to block access.
This delayed the approval and release of lower-cost alternatives, keeping drug prices high.
What the CREATES Act Does:Allows generic and biosimilar manufacturers to request samples of covered products from brand-name companies. If the brand refuses without a legitimate reason, the generic company can sue in federal court. Courts can order the brand to provide samples and may impose financial penalties for delays.
How CPA and the CREATES Act Work Together.Covered Product Authorization means a drug or biologic has full FDA approval and is on the market.
The CREATES Act ensures that generic and biosimilar makers can access those covered products for testing: so, they can make affordable versions.
- This promotes competition, improves drug access, and reduces costs for patients.
- Without CPA, unsafe or untested products could enter the market.
- Without the CREATES Act, brand-name companies could unfairly delay the competition.
- Together, they ensure a balance between innovation, safety, and affordable access to medicine.
Regulatory Success with Masuu: CPA and CREATES Act Expertise
At Masuu, we are a trusted global regulatory and quality consulting agency dedicated to helping pharmaceutical and biotech companies succeed in product development and compliance. Our expertise includes end-to-end support for FDA Covered Product Authorization (CPA) and the CREATES Act process.
We assist you with:- Navigating the regulatory pathway for FDA-approved drugs and biologics
- Ensuring full compliance with CPA requirements for safe and effective product approval
- Facilitating access to reference product samples under the CREATES Act for generic and biosimilar development
- Providing strategic guidance for faster approvals and market entry
- Delivering tailored solutions for quality assurance, documentation, and FDA submissions
Partner with Masuu to streamline your development, reduce time-to-market, and ensure global regulatory success.
